The VISIBLE Psoriasis Clinical Study
The VISIBLE Study is now enrolling people with skin of color to research an investigational medicine.
Don’t be afraid to make yourself seen

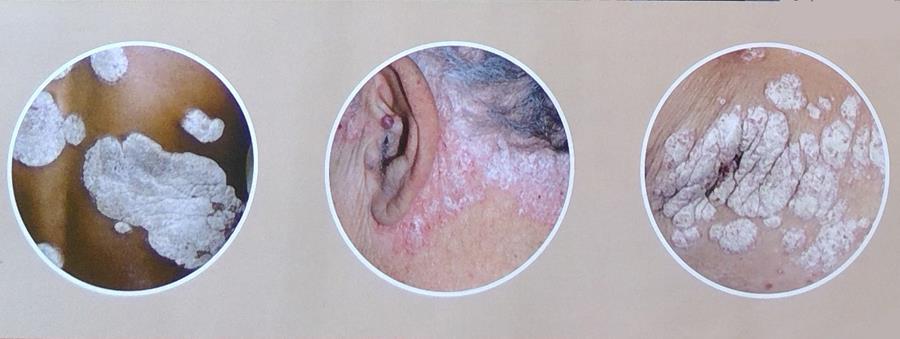
The VISIBLE Study is now enrolling people with skin of color to research an investigational medicine for scalp and body plaque psoriasis.
You may be eligible to participate if:
- You are 18 years or older
- You self identify as non-white
- You have been diagnosed with or think you may have scalp or body plaque psoriasis
The commitment for the study is about 2 years.
Qualified persons will receive study-required medical care and the active investigational medicine or placebo at no cost. The study will not pay for any other medical care or current medication(s) needed to support your daily health care routine. You may leave the study at anytime.
For More Information:
If you are interested in participating, please contact Darst Dermatology and ask for Shanna Miller at 704-321-3376 ext 1017 or email her at: [email protected]

More about the study:
Janssen Pharmaceutical Companies of Johnson & Johnson initiated VISIBLE, a first-of-its-kind, large-scale prospective clinical study dedicated to people of color living with moderate to severe plaque and/or scalp PsO.
VISIBLE will further evaluate the efficacy and safety of TREMFYA® (guselkumab) in people of color to generate additional data and provide valuable information about disease burden and the psoriatic disease patient journey in this population.
TREMFYA has a well-established safety and efficacy profile across a broad patient population of adults with moderate to severe PsO. However, there is still a pressing need for more data in people of color,4 given that over the course of about 20 years, the majority of Phase 3 PsO clinical trials (across topical, oral, and biologic therapies) have enrolled predominantly white participants (86 percent).5 The VISIBLE study is guided by the company’s strong commitment to bioethicsa and designed to help promote more diverse, equitable and inclusive clinical research in PsO through new approaches to enrollment and retention, broader community engagement and new data components.
